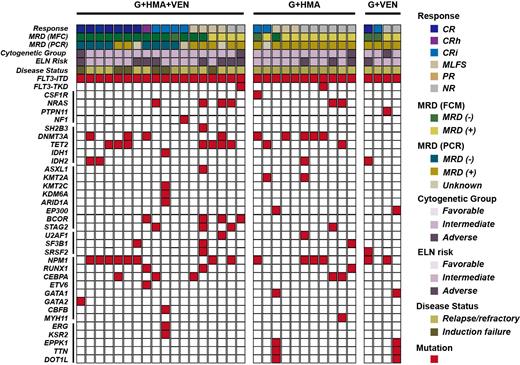
Gilteritinib, a potent FMS-like tyrosine kinase 3 (FLT3) inhibitor, was approved for relapsed/refractory (R/R) FLT3 mutated acute myeloid leukemia (AML) patients but still showed limited efficacy. Here, the efficacy and safety of different gilteritinib-based combination therapy (gilteritinib plus hypomethylating agent [HMA] and venetoclax, G+HMA+VEN; gilteritinib plus HMA, G+HMA; gilteritinib plus venetoclax, G+VEN) in 33 R/R FLT3 mutated AML patients were retrospectively analyzed. Composite complete response (CRc) rate (complete response [CR]+CR with partial hematologic recovery [CRh]+CR with incomplete blood count recovery [CRi]), modified CRc rate (mCRc, CRc+morphologic leukemia-free state [MLFS]), overall survival (OS), duration of remission (DOR), and adverse events were analyzed and compared.The CRc and mCRc rate were 66.7% (12/18) and 88.9% (16/18) in patients who received G+HMA+VEN, which was higher compared with that in G+HMA (CRc: 18.2%, 2/11; mCRc: 45.5%, 5/11) or G+VEN (CRc: 50.0%, 2/4; mCRc: 50.0%, 2/4). The median OS for G+HMA+VEN, G+HMA, G+VEN treatment was not reached, 145.5 days, and 231.0 days, respectively (G+HMA+VEN vs G+HMA, p=0.011). The median DOR for G+HMA+VEN, G+HMA, G+VEN treatment was not reached, 82.0 days, and 77.0 days, respectively (G+HMA+VEN vs G+VEN, p=0.040). Four patients in G+HMA+VEN group received allogenic hematopoietic stem cell transplantation (alloHSCT) after remission exhibited prolonged OS and DOR. The most common grade 3/4 adverse events were cytopenia, febrile neutropenia and pulmonary infection, there were no difference among three groups.In conclusion, our data demonstrated promising response of G+HMA+VEN combination therapy in R/R FLT3 mutated AML patients, and it may consider as an effective bridge to transplantation.
Disclosures
No relevant conflicts of interest to declare.